
Capacity Shortages Unlikely To Hold Back Cell And Gene Therapies
The pharmaceutical industry's manufacturing capacity for cell and gene therapies is under pressure from an ever-increasing number of life sciences companies wanting to exploit rare expertise in the sector, but CMOs and government-backed facilities are rapidly coming on-stream, hoping to develop local clusters of companies to deliver the complete "living medicines" supply chain.

The Rise Of The Digital Factory – Sanofi's Story
As digital continues to take hold within the pharma industry's clinical activities, Scrip explores how technology enhancements are being put into practice in another area of the drug development cycle – manufacturing.

Hurricane Maria Tests Pharma Business Continuity Plans
Puerto Rico's slow recovery from Hurricane Maria provides insight into what happens when disaster strikes a node of the global pharmaceutical manufacturing network. Initial efforts to ward off US drug shortages focus on generic injectables that can't support expensive business continuity plans – but even critical prescription brands could be threatened if the US territory's power grid remains off line too long.
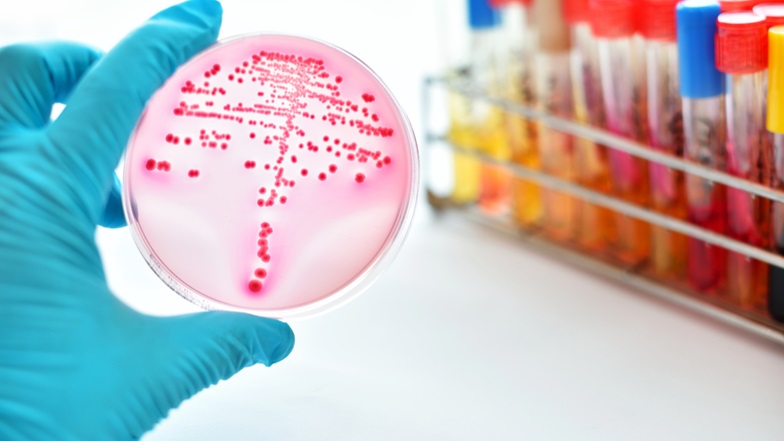
How To Find And Remedy Data Integrity Lapses In Microbiology Labs
Analytical chemistry labs may get all the attention when it comes to the search for data integrity failures, but FDA and industry experts say not to overlook the possibility of such failures in microbiology labs. Just keep in mind that the highly manual operations in these labs calls for a different approach to auditing.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.

