Other Therapy Areas
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.
Aesthetics
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Revance Wins First Daxxify Therapeutic Indication, Plans Limited Initial Rollout
Similar to its post-approval strategy in aesthetics less than a year ago, Revance will initially target a small number of doctors as it Daxxify to market in the US for cervical dystonia with a broader launch in 2024.

BiologicsMD Uses Unique Fusion Proteins To Treat Alopecia Safely
Emerging Company Profile: The US biotech is developing a series of recombinant fusion proteins to tackle alopecia and other diseases without supressing the immune system.

Finance Watch: Brent Saunders Plots Return To Deal-Making With A SPAC IPO
Public Company Edition: The former Allergan CEO is taking a special purpose acquisition corporation public to generate $460m for deals in familiar biopharma niches. Also, IPOs are expected to keep up a robust pace in the fall and Albireo raises $160m on the success of its drug for pediatric liver diseases.

ITC Takes Medytox’s Hand In US Botulinum Case, Bans Daewoong’s Imports
USITC’s initial decision in the Medytox and Daewoong botulinum dispute is favorable to Medytox and poised to take a toll on the Korean firm and partner Evolus as they face a 10-year ban on imports of their product to the US.
Blood and Coagulation Disorders
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Abecma Or Bust: 2seventy Sells Final Research Program To Novo
2seventy bio sold full rights to a hemophilia A program and in vivo gene editing technology to partner Novo Nordisk.

China Phase III Results Boost Global Prospects For HUTCHMED ITP Drug
The Phase III ESLIM-01 study with HUTCHMED’s Syk inhibitor sovleplenib met its primary endpoint, demonstrating a 48.4% durable response rate for chronic immune thrombocytopenia, significantly higher than placebo. Global development appears to be accelerating and a multinational dose-finding Phase Ib study has also been initiated.

Agios Shows Pyrukynd Can Reduce Transfusions In Thalassemia
Having already posted Phase III success in non-transfusion dependent patients, Agios’s Pyrukynd now has shown the ability to reduce transfusions in alpha and beta thalassemia patients.

EHA Preview: ASCO Overlap Gives Smaller Companies Chance To Shine
Cargo Therapeutics has got an early boost from its abstract to be presented the European meeting, while Shattuck has suffered on safety doubts for its CD47 inhibitor.
Dental Oral
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Start-up AFYX Applies Ultra-Sticky Patch Technology To Underserved Oral Mucosal Disease
Emerging Company Profile: Patients with oral lichen planus suffer from painful lesions in the mouth that are hard to treat with topical steroids, leaving room for development of a new delivery method for the established clobetasol.
Dermatology
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Quick Listen: Scrip's Five Must-Know Things
In this week's podcast edition of Five Must-Know Things: the outlook for 2025 launches; Merck confident in new pneumococcal vaccine; multiple US setbacks for Japanese firms; Stock Watch on Moderna missteps; and Sun’s North America head talks plans and strategy.

Japan Academia Progresses Novel Melanoma Drug Despite Pharma 'Reluctance'
Academia-developed novel oral PAI-1 inhibitor in combination with Opdivo showed a strong response rate in PD-1 treated melanoma patients with certain disease characteristics and could represent a new breakthrough for a class with no clinical stage competitors.

InflaRx Undaunted By Dermatology Rivals
The group says INF904 can make billions in two highly competitive skin diseases, but this looks optimistic.

Soterios Topical Could Take On JAK Class In Alopecia Areata
The UK biotech unveils Phase II data showing an ability to reduce severity of mild-to-moderate AA, with full hair restoration in some patients. If approved, STS-01 will compete with Olumiant and Litfulo.
Ear
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Regeneron Gene Therapy Reaches New Heights In Restoring Hearing
The US biotech’s DB-OTO has made headlines worldwide after a baby with profound genetic deafness experienced improved hearing to normal levels within 24 weeks after a single intracochlear injection.

Fennec Finds Ex-US Commercial Partner For Pedmark In UK’s Norgine
Norgine will market Pedmark, indicated to reduce risk of chemotherapy-related hearing loss in pediatric cancer, in the EU and UK. It will seek additional approvals in Australia and New Zealand.

Acousia To Refresh Stagnant Hearing Loss Space With New Potassium Channel Approach
The German biotech plans to prevent chemotherapy-induced hearing loss and treat chronic hearing loss by targeting a potassium channel in the ear’s sensory hair cells as it tackles a widespread but side-lined area.
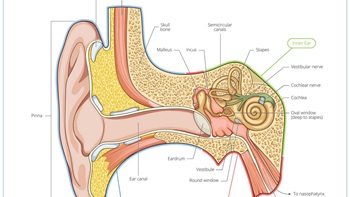
Lilly Builds On Gene Therapy Focus With Akouos Takeout
With the planned $487m acquisition of sensorineural-focused Akouos, Lilly makes its second gene therapy M&A play in two years, on top of partnering/financing activity in the space.
Ear Nose Throat
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.
Regeneron Strikes A CHORD With Deafness Gene Therapy
A very early success with a gene therapy for a rare form of deafness puts Regeneron ahead of Lilly and Sensorion.

Fennec Prepared For Market As Pedmark Finally Gets FDA Nod
After successive CRLs in 2020 and 2021, the drug gained a broad label for hearing loss in children treated with cisplatin for localized, non-metastatic solid tumors.

Optinose Looking For A Partner To Optimize Big Opportunity In Chronic Sinusitis
With positive Phase III data for its Xhance (fluticasone propionate) nasal spray in chronic sinusitis, Optinose believes it is poised to expand the target market for the drug 10-fold.

Budesonide CRL Another Setback For Takeda’s ‘Wave 1’ Pipeline
Setback for company's near-term launch goals and US patients with rare esophageal inflammatory condition as regulator asks for additional clinical data.
Gastrointestinal
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Ensho Joins Race To Develop An Oral Integrin Inhibitor For IBD
Ensho Therapeutics is raising series A cash to develop Phase II-ready NSHO-101 (EA1080), a third oral ɑ4β7 integrin inhibitor attempting to take on Takeda’s blockbuster injectable Entyvio.

CalciMedica Eyes Success In Pancreatitis, Acute Kidney Injury
Phase IIb data for Auxora in an acute pancreatitis subpopulation is expected to set up the CRAC channel inhibitor for Phase III in that indication while suggesting positive read-through for AKI.

J&J Heats Up IL-23 Inflammatory Bowel Space With Tremfya Crohn's US Filing
AbbVie's rival interleukin-23 drug Skyrizi is now approved in the US for both principal types of inflammatory bowel disease – ulcerative colitis and Crohn’s disease. Tremfya may not be too far behind.

AbbVie Doubles Down In IBD With Skyrizi Ulcerative Colitis Approval
The US major's blockbuster is already well established as a treatment for Crohn’s disease but the latest thumbs-up from the US FDA means Skyrizi is the first interleukin-23 inhibitor approved for both principal types of inflammatory bowel disease.
Gynecology Urology
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

SCOTUS' Mifepristone Decision Sets High Bar For US FDA Suits
The unanimous decision that the Alliance for Hippocratic Medicine lacks standing to challenge the FDA’s relaxation of the abortion pill REMS leaves unclear whether the court would have deferred to the agency’s expertise on the merits.

Bayer’s Menopause Drug Has An Edge But Still Much To Prove
Phase III results suggest Bayer’s elinzanetant has a safety and efficacy advantage over its Astellas rival in reducing hot flashes, but investors are still not convinced the drug will be a blockbuster.

Odd Coupling: J&J, RallyBio Collaborate As Only Firms Focused On FNAIT
While not co-developing each other’s drugs, J&J and financially troubled RallyBio are working on complementary approaches to the rare disease fetal and neonatal alloimmune thrombocytopenia.

Genmab Adds To Sector ADC Buying Spree With $1.8bn ProfoundBio Acquisition
The buyout reflects the Danish biotech’s plans to expand in oncology drug development and commercialization.
Liver Hepatic
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Akero Shows More Strong Fibrosis Data, But Analysts Cite Safety Worry
Akero is developing weekly injectable efruxifermin for MASH with solid 96-week efficacy data and three Phase III studies underway. But will it find a treatment niche in a competitive space?

Ipsen Gets Ahead Of The Qirv in Cholangitis
Iqirvo gains accelerated approval, but Gilead’s seladelpar is probably only two months behind.

EASL: Sagimet Pill Is Overlooked But Could Make A Splash In MASH
While injectables have had all the limelight, some analysts see a bright future for Sagimet’s oral denifanstat, potentially as a backbone therapy in combination with other treatments for MASH.

Viking Bolsters MASH Case With 52-Week Histology Data
Viking will look to plan a Phase III program with the FDA after showing 52-week success on MASH resolution and fibrosis reduction with VK2809. The firm still hopes to move forward with a partner.
Ophthalmic
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Apellis To Try Again After EMA Rejects Geographic Atrophy Drug
The delayed decision from the CHMP is another rejection for Syfovre but Apellis still hopes the regulator may be convinced in a second review later this year.

Multiple Stargardt Contenders Progress In Japan Despite Small Market
With no approved therapies globally for Stargardt's, competition in the indication is increasing in Japan, where Kubota Vision is seeking for conditional approval for emixustat, while Belite Bio’s contender tinlarebant has received Sakigake designation as a local Phase III program continues.

Skye Bioscience Looks To Build Appetite For CB1 Obesity Drug
The company will take on Novo Nordisk in reviving the CB1 inhibitor mechanism to develop a more tolerable long-term weight loss drug, after exiting ophthalmology.

Oculis Looks To Move Dry Eye Candidate To Phase III Despite Mixed Dataset
Oculis’s licaminlimab showed ability to treat a sign of dry eye disease in a genetic subpopulation, but data in the fuller Phase IIb trial enrollment were less impressive.
Orthopedics
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Lilly In No Hurry To Share Results From Muscle-Building And Obesity Combination
A new muscle-building front is opening in the fight against obesity, with Lilly among the leaders. But it plans to remain tight-lipped on upcoming Phase II data for its myostatin inhibitor in combination with Novo’s Wegovy for fear of playing into the hands of its rival.

Ultragenyx Data De-Risk Phase III Trial Of Setrusumab
The Mereo-partnered drug is in pivotal development for osteogenesis imperfecta. Ultragenyx also recently reported positive Phase III data for a rare disease gene therapy.

Bridgebio Ready To Challenge BioMarin In Achondroplasia
The company sees a $5bn opportunity to treat children with achondroplasia and the less severe growth disorder variant, hypochondroplasia.

Dyne Hopes DM1 And DMD Mid-Stage Data Can Lead To Accelerated Approval
Phase I/II data for its ADC candidates in myotonic dystrophy type 1 and Duchenne muscular dystrophy indicate potential for biomarker-based accelerated approval in both diseases, Dyne asserts.
Poisoning
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

SERB Pays $800m For Boston Scientific Specialty Pharma Business
The sale of BTG Specialty Pharmaceuticals to two affiliates of European specialty firm SERB leaves Boston Scientific with BTG’s interventional oncology and vascular products.

£80m To Tackle Snakebite Is A Wellcome Boost
Two major initiatives have been announced which hope to revolutionize the treatment of snakebites, which claims the lives of up to 138,000 people a year.

Indivior Pact Buys Addex Time For Progressing Dipraglurant And Pipeline
Addex Therapeutics says its licensing deal with Indivior to develop and commercialize its investigative addiction therapy ADX71441 'buys time' to find resources for progressing its own pipeline.
US Capitol Capsule: Move over Ebola! Fake drugs fight needs global attention, too
Much of the attention at the 2015 World Health Assembly (WHA) in Geneva, Switzerland later this month is expected to be focused on reforming the World Health Organization (WHO) and the failures by the international public health agency in responding to the ongoing Ebola epidemic in West Africa.
Renal
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Cagrisema Leads The Charge For 2025
2030 sales forecasts for Novo’s obesity hope are an order of magnitude larger than its closest rival among 2025’s expected debutantes.

Quick Listen: Scrip's Five Must-Know Things
In this week's podcast edition of Five Must-Know Things: ASCO preview; Asahi Kasei’s US acquisition; Novartis aims for renal disease dominance; paying for gene therapies; and US BIOSECURE Act diluted.

Asahi Kasei Furthers Its US, Health Care Growth With $1.1bn Calliditas Buy
Asahi Kasei established a US presence and expertise in renal disease when it bought Veloxis in 2020 and will expand in both areas with Calliditas’s Tarpeyo for immunoglobulin A nephropathy (IgAN).

Novartis Aims To Dominate Rare Renal Disorders
Fabhalta and atrasentan are odds-on for approval in C3 glomerulopathy and IgA nephropathy, respectively, but commercial success might be harder to come by.
Wound Healing Tissue Repair
Are you sure you'd like to remove this alert? You will no longer receive email updates about this topic.

Conditional Global-First Nod For SanBio's Cell Therapy for TBI
SanBio’s lead cell therapy asset has been on a bumpy journey to its global-first approval and while a nod has now come in Japan in a high-need indication, a commercial launch is conditional on additional data to establish product equivalence and manufacturing consistency.

Healios Acquires Bankrupt Athersys, Expands Cell Therapy R&D Programs
Tokyo-based Healios will lead the global clinical studies for Athersys’ cell therapy MultiStem for ARDS and start US Phase II trials for trauma following their long-term collaboration.

Swiss R&D Innovation: A Tale Of Two Firms Developing Cell Therapies For Wounds
While perhaps best known for historical achievements in immuno-oncology and CNS disorders, the Swiss biotech sector is slowly cultivating a new area of expertise in dermatology. Scrip spoke with two firms applying cell therapy technologies to the challenging area of chronic and acute wounds.

Vertex’s Type 1 Diabetes Cell Therapy Gets A Boost – And Some Competition
Vertex has invested heavily in advancing its stem-cell based candidates for type 1 diabetes, but Sernova has inched ahead in the race to bring a functional cure for the condition to patients.
You must sign in to use this functionality
Authentication.SignIn.HeadSignInHeader
Email Article
All set! This article has been sent to my@email.address.
All fields are required. For multiple recipients, separate email addresses with a semicolon.
Please Note: Only individuals with an active subscription will be able to access the full article. All other readers will be directed to the abstract and would need to subscribe.